Clinicopathological assessment of cancer/testis antigens NY‑ESO‑1 and MAGE‑A4 in osteosarcoma
Accepted: 16 June 2022
HTML: 17
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
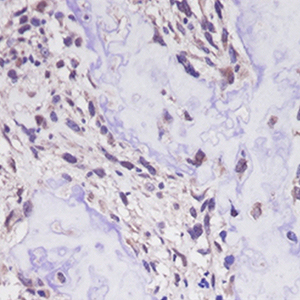
The cancer/testis antigens (CTAs), New York esophageal squamous cell carcinoma-1 (NY-ESO-1) and melanoma antigen gene (MAGE)-A4 are normally restricted to male germ cells but are aberrantly expressed in several cancers. Considering the limited information regarding their significance in osteosarcoma (OS), the purpose of this study was to determine the clinical significance of NY-ESO-1 and MAGE-A4 expression in OS. Nine patients with OS treated at Kindai University Hospital were included in the study. The median age was 27 years, and median follow-up period was 40 months. The specimens obtained at the time of biopsy were used to perform immunostaining for NY-ESO, MAGE-A4, p53, and Ki-67. The positive cell rates and positive case rates of NY-ESO, MAGE-A4, p53, and Ki-67 were calculated. The correlation between the positive cell rate of immunohistochemical markers was also calculated. The correlation between the positive cell rate of NY-ESO-1 or MAGE-A4 and tumor size or maximum standardized uptake (SUV-max) was also determined. The positive cell rates of NY-ESO-1 or MAGE-A4 in continuous disease-free (CDF) cases were also compared with those in alive with disease (AWD) or dead of disease (DOD) cases. The average positive cell rates of NY-ESO, MAGEA4, p53, and Ki-67 were 71.7%, 85.1%, 16.2%, and 14.7%, and their positive case rates were 33.3%, 100%, 44.4%, and 100%, respectively. The positivity rates of NY-ESO-1 and p53 were strongly correlated, whereas those of NY-ESO-1 and Ki-67 were moderately correlated. The MAGE-A4 and p53 positivity rates and the MAGE-A4 and Ki-67 positive cell rates were both strongly correlated. The NY-ESO-1 and MAGE-A4 positivity rates were moderately correlated. The positive correlation between the NY-ESO-1 positive cell rate and tumor size was medium, and that between the MAGE-A4 positivity rate and SUV-max was very strong. There was no significant difference in the positive cell rates of NY-ESO-1 or MAGE-A4 between CDF cases and AWD or DOD cases. Overall, our results suggest that NY-ESO-1 and MAGE-A4 may be involved in the aggressiveness of OS.
Ethics Approval
This study was approved by the Ethics Committee of Kindai University Hospital (approval number: R03-021, Osaka, Japan; approval date: April 27, 2021)How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2022.3377
https://doi.org/10.4081/ejh.2022.3377






