High expression of both desmoplastic stroma and epithelial to mesenchymal transition markers associate with shorter survival in pancreatic ductal adenocarcinoma
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Accepted: 9 February 2022
Authors
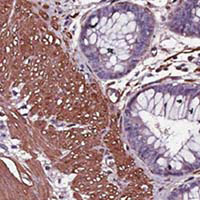
Desmoplastic stroma (DS) and the epithelial-to-mesenchymal transition (EMT) play a key role in pancreatic ductal adenocarcinoma (PDAC) progression. To date, however, the combined expression of DS and EMT markers, and their association with variations in survival within each clinical stage and degree of tumor differentiation is unknown. The purpose of this study was to investigate the association between expression of DS and EMT markers and survival variability in patients diagnosed with PDAC. We examined the expression levels of DS markers alpha smooth muscle actin (α-SMA), fibronectin, and vimentin, and the EMT markers epithelial cell adhesion molecule (EPCAM), pan-cytokeratin, and vimentin, by immunohistochemistry using a tissue microarray of a retrospective cohort of 25 patients with PDAC. The results were examined for association with survival by clinical stage and by degree of tumor differentiation. High DS markers expression -α-SMA, fibronectin, and vimentin- was associated with decreased survival at intermediate and advanced clinical stages (p=0.006-0.03), as well as with both poorly and moderately differentiated tumor grades (p=0.01-0.02). Interestingly, the same pattern was observed for EMT markers, i.e., EPCAM, pan-cytokeratin, and vimentin (p=0.00008-0.03). High expression of DS and EMT markers within each clinical stage and degree of tumor differentiation was associated with lower PDAC survival. Evaluation of these markers may have a prognostic impact on survival time variation in patients with PDAC.
Supporting Agencies
national science and technology council, coordination of health researchPrograma de Doctorado de Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM), Mexico City, Mexico
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.