Postnatal development of thalamic reticular nucleus projections to the anterior thalamic nuclei in rats
Accepted: 26 February 2022
HTML: 24
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
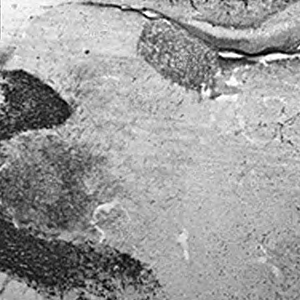
The thalamic reticular nucleus (TRN) projects inhibitory signals to the thalamus, thereby controlling thalamocortical connections. Few studies have examined the development of TRN projections to the anterior thalamic nuclei with regard to axon course and the axon terminal distributions. In the present study, we used parvalbumin (PV) immunostaining to investigate inhibitory projections from the TRN to the thalamus in postnatal (P) 2- to 5-week-old rats (P14–35). The distribution of PV-positive (+) nerve fibers and nerve terminals markedly differed among the anterior thalamic nuclei at P14. Small, beaded nerve terminals were more distributed throughout the anterodorsal nucleus (AD) than in the anteroventral nucleus (AV) and anteromedial nucleus (AM). PV+ fibers traveling from the TRN to the AD were observed in the AV and AM. Nodular nerve terminals, spindle or en passant terminals, were identified on the axons passing through the AV and AM. At P21, axon bundles traveling without nodular terminals were observed, and nerve terminals were distributed throughout the AV and AM similar to the AD. At P28 and P35, the nerve terminals were evenly distributed throughout each nucleus. In addition, DiI tracer injections into the retrosplenial cortex revealed retrogradely-labeled projection neurons in the 3 nuclei at P14. At P14, the AD received abundant projections from the TRN and then projected to the retrosplenial cortex. The AV and AM seem to receive projections with distinct nodular nerve terminals from the TRN and project to the retrosplenial cortex. The projections from TRN to the AV and AM with nodular nerve terminals at P14 are probably developmental-period specific. In comparison, the TRN projections to the AD at P14 might be related to the development of spatial navigation as part of the head orientation system.
Ethics Approval
This study is approved under the guidelines of the official Japanese regulations for research on animals in the Yokohama City University (approval NO. F-A-16-064)Supporting Agencies
Fundamental Research Funding of Yokohama City UniversityHow to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2022.3370
https://doi.org/10.4081/ejh.2022.3370






