Seasonal changes in the expression of PACAP, VPAC1, VPAC2, PAC1 and testicular activity in the testis of the muskrat (Ondatra zibethicus)
Accepted: 20 April 2022
HTML: 59
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
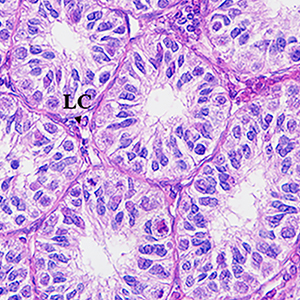
Pituitary adenylate cyclase-activating polypeptide (PACAP) plays an important role in the steroidogenesis and spermatogenesis in the testis through its receptors PAC1, VPAC1, and VPAC2. In this study, we investigated the seasonal expressions of PACAP, PAC1, VPAC1, VPAC2, luteinizing hormone receptor (LHR), follicle stimulating hormone receptor (FSHR), steroidogenic acute regulatory protein (StAR), 3β-hydroxysteroid dehydrogenase (3β-HSD), and CYP17A1 in the testis of the male muskrat during the breeding season and the non-breeding season. Histologically, we found the presence of Leydig cells, Sertoli cells and all kinds of germ cells in the testis during the breeding season but only Leydig cells, Sertoli cells, spermatogonia and primary spermatocyte during the non-breeding season. The immunohistochemical localizations of PACAP and VPAC1 were identified in the Leydig cells, spermatogonia and spermatozoa during the breeding season while only in Leydig cells and spermatogonia during the non-breeding season, and PAC1 and VPAC2 were localized in the Leydig cells in both seasons, in which LHR, StAR, 3β-HSD and CYP17A1 were also expressed. Meanwhile, protein and mRNA expression levels of PACAP, PAC1, VPAC1, VPAC2, LHR, FSHR, StAR, 3β-HSD and CYP17A1 in the testis during the breeding season were significantly higher than those during the non-breeding season. These results suggested that PACAP may involve in the regulation of, steroidogenesis and spermatogenesis via an endocrine, autocrine or paracrine manner in the testis of the muskrat.
Ethics Approval
All animals were treated according to the Policy on the Care and Use of Animals by the Ethical Committee and Animal Welfare Committee and all procedures were approved by Beijing Forestry University, China (EAWC_BJFU_2022003)How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2022.3398
https://doi.org/10.4081/ejh.2022.3398






