Identification of abnormally high expression of POGZ as a new biomarker associated with a poor prognosis in osteosarcoma
Accepted: 9 July 2021
Supplementary: 97
HTML: 13
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
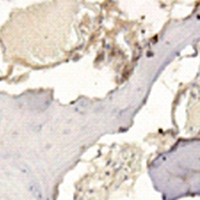
Osteosarcoma (OS) is the most prevalent malignant bone tumor in children and young adults. There is an urgent need for a novel biomarker related to the prognosis of OS. We performed a meta-analysis incorporating six independent datasets and performed a survival analysis with one independent dataset GSE21257 in the GEO database for gene screening. The results revealed that one potential biomarker related to OS survival, POGZ was the most significantly upregulated gene. We also verified that the POGZ was overexpressed in clinical samples. The survival analysis revealed that POGZ is associated with a poor prognosis in OS. Moreover, flow cytometry analysis of isolated OS cells demonstrated that OS cells were arrested in the G1 phase after POGZ knockdown. The RNA-seq results indicated that POGZ was co-expressed with CCNE1 and CCNB1. Pathway analysis showed that genes associated with high expression levels of POGZ were related to the cell cycle pathway. A cell model was constructed to detect the effects of POGZ. After POGZ knockdown, OS cell proliferation, invasion and migration were all decreased. Therefore, POGZ is an important gene for evaluating the prognosis of OS patients and is a potential therapeutic target.
Supporting Agencies
Natural Science Research Program of Jiangxi (No. 20202ACBL206012. to XC), National Natural Science Foundation of China (No. 81660357 to XC), National Natural Science Foundation of China (No. 81860397 to XC)How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2021.3264
https://doi.org/10.4081/ejh.2021.3264






