Colorimetric and fluorescent TRAP assays for visualising and quantifying fish osteoclast activity
Accepted: 8 March 2022
HTML: 61
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
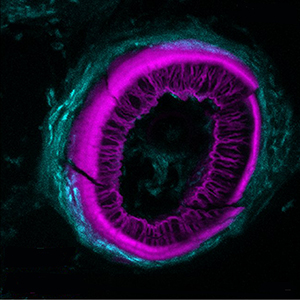
Histochemical detection of tartrate-resistant acid phosphatase (TRAP) activity is a fundamental technique for visualizing osteoclastic bone resorption and assessing osteoclast activity status in tissues. This approach has mostly employed colorimetric detection, which has limited quantification of activity in situ and co-labelling with other skeletal markers. Here we report simple colorimetric and fluorescent TRAP assays in zebrafish and medaka, two important model organisms for investigating the pathogenesis of bone disorders. We show fluorescent TRAP staining, utilising the ELF97 substrate, is a rapid, robust and stable system to visualise and quantify osteoclast activity in zebrafish, and is compatible with other fluorescence stains, transgenic lines and antibody approaches. Using this approach, we show that TRAP activity is predominantly found around the base of the zebrafish pharyngeal teeth, where osteoclast activity state appears to be heterogeneous.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2022.3369
https://doi.org/10.4081/ejh.2022.3369






