Phosphorylation mutation impairs the promoting effect of spastin on neurite outgrowth without affecting its microtubule severing ability
Accepted: 27 December 2022
HTML: 27
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
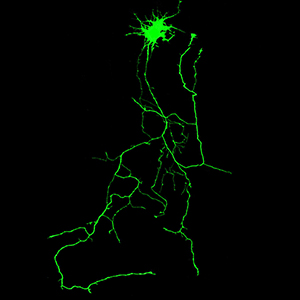
Spastin, a microtubule-severing enzyme, is known to be important for neurite outgrowth. However, the role of spastin post-translational modification, particularly its phosphorylation regulation in neuronal outgrowth, remains unclear. This study aimed to investigate the effects of eliminating spastin phosphorylation on the neurite outgrowth of rat hippocampal neurons. To accomplish this, we constructed a spastin mutant with eleven potential phosphorylation sites mutated to alanine. The phosphorylation levels of the wildtype spastin (WT) and the mutant (11A) were then detected using Phos-tag SDS-PAGE. The spastin constructs were transfected into COS7 cells for the observation of microtubule severing, and into rat hippocampal neurons for the detection of neuronal outgrowth. The results showed that compared to the spastin WT, the phosphorylation levels were significantly reduced in the spastin 11A mutant. The spastin mutant 11A impaired its ability to promote neurite length, branching, and complexity in hippocampal neurons, but did not affect its ability to sever microtubules in COS7 cells. In conclusion, the data suggest that mutations at multiple phosphorylation sites of spastin do not impair its microtubule cleavage ability in COS7 cells, but reduce its ability to promote neurite outgrowth in rat hippocampal neurons.
Ethics Approval
All animal experiments were conducted in accordance with the Regulations of the People's Republic of China on the Administration of Laboratory Animals and were reviewed and approved by the Ethics Committee of Jinan UniversitySupporting Agencies
National Natural Science Foundation of ChinaHow to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2023.3594
https://doi.org/10.4081/ejh.2023.3594






