An ex vivo experimental system to track fluorescent nanoparticles inside skeletal muscle
Accepted: 14 December 2022
HTML: 23
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
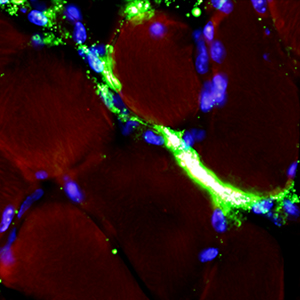
The development of novel nanoconstructs for biomedical applications requires the assessment of their biodistribution, metabolism and clearance in single cells, organs and entire organisms in a living environment. To reduce the number of in vivo experiments performed and to refine the methods used, in accordance with the 3Rs principle, this work proposes an ex vivo experimental system to monitor, using fluorescence microscopy, the distribution of nanoparticles in explanted murine skeletal muscle maintained in a bioreactor that can preserve the structural and functional features of the organ for long periods of time. Fluorescently-labelled liposomes and poly(lactide-co-glycolide) (PLGA)-based nanoparticles were injected into the intact soleus muscle (in the distal region close to the tendon) immediately after explants, and their distribution was analysed at increasing incubation times in cross cryosections from the proximal region of the belly. Both nanocarriers were clearly recognized in the muscle and were found to enter and migrate inside the myofibres, whereas their migration in the connective tissue seemed to be limited. In addition, some fluorescent signals were observed inside the macrophages, demonstrating the physiological clearance of the nanocarriers that did not enter the myofibres. Our ex vivo system therefore provides more information than previous in vitro experiments on cultured muscle cells, highlighting the need for the appropriate functionalization of nanocarriers if myofibre targeting is to be improved.
Ethics Approval
This study was approved by the Italian Ministry of HealthSupporting Agencies
This work did not receive specific funding and was performed thanks to intramural funds to M.M.Present address: Department of Health Sciences, University of Piemonte Orientale “A. Avogadro”, Novara, Italy
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2023.3596
https://doi.org/10.4081/ejh.2023.3596






