Immunofluorescence signal intensity measurements as a semi-quantitative tool to assess sarcoglycan complex expression in muscle biopsy
Accepted: 20 June 2022
HTML: 17
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
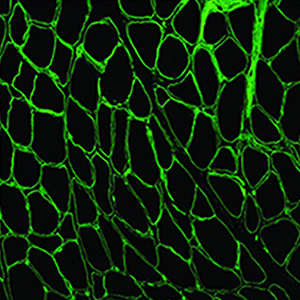
Sarcoglycanopathies are highly heterogeneous in terms of disease progression, muscular weakness, loss of ambulation and cardiac/respiratory involvement. Their clinical severity usually correlates with the residual protein amount, which makes protein quantification extremely relevant. Sarcoglycanopathy diagnosis is genetic, but skeletal muscle analysis - by both immunohistochemistry and Western blot (WB) - is still mandatory to establish the correct diagnostic process. Unfortunately, however, WB analysis cannot be performed if the bioptic specimen is scarce. This study provides a sensitive tool for semi-quantification of residual amount of sarcoglycans in patients affected by sarcoglycanopathies, based on immunofluorescence staining on skeletal muscle sections, image acquisition and software elaboration. We applied this method to eleven sarcoglycanopathies, seven Becker muscular dystrophies and four age-matched controls. Fluorescence data analysed in patients and compared to age-matched controls showed a significant reduction of the mutated sarcoglycan expression and a variable reduction of the other sarcoglycans. Fluorescence normalized data analysed in relation to the age of onset of the disease, showed a negative correlation of α-sarcoglycan fluorescent signal versus fibrosis in patients with an early age of onset and a negative correlation between δ-sarcoglycan signal and fibrosis in both intermediate and late age of onset groups. The availability of a method that allows objective quantification of the sarcolemmal proteins, faster and less consuming than WB analysis and able to detect low residual sarcoglycan expression with great sensitivity, proves useful to better define both patient prognosis and expected disease evolution. The proposed method could be employed also to monitor the efficacy of therapeutic interventions and during clinical trials.
Neurology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan; Dino Ferrari Centre, Department of Pathophysiology and Transplantation (DEPT), University of Milan
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2022.3418
https://doi.org/10.4081/ejh.2022.3418






