Umbilical cord mesenchymal stem cells inhibited inflammation of bronchial epithelial cells by regulating Hedgehog pathway
Accepted: 28 November 2023
HTML: 2
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
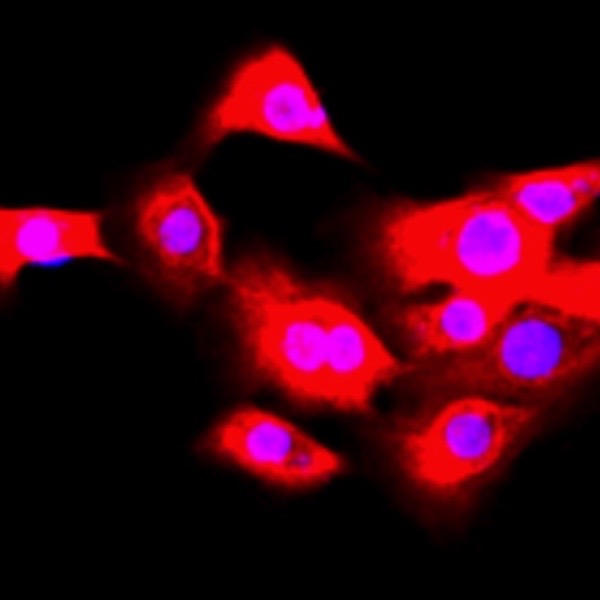
This study aimed to explore the role and mechanism of umbilical cord mesenchymal stem cells (UCMSCs) in regulating inflammation of bronchial epithelial cells. Transforming growth factor beta-1 (TGF-β1) was used to induce inflammation in human bronchial epithelial cells. Cell proliferation was detected through CCK8 and cell apoptosis was detected by Annexin V and propidium iodide double staining. E-cadherin and α-smooth muscle actin (α-SMA) were detected by immunofluorescence, and tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) in culture medium supernatant were detected by ELISA. The expression of E-cadherin, α-SMA, Sonic hedgehog (Shh), Gli1 and Snail was detected by Western blot analysis. Compared with the control group, bronchial epithelial cells treated with TGF-β1 showed significantly decreased proliferation, increased apoptosis, increased secretion of TNF-α and IL-6, increased expression of α-SMA, Shh, Gli1 and Snail and decreased E-cadherin expression. However, co-culture with UCMSCs inhibited TGF-β1-induced changes in human bronchial epithelial cell proliferation, apoptosis, secretion of TNF-α and IL-6 and activation of the Hedgehog pathway. In conclusion, UCMSCs have protective effects on TGF-β1-induced inflammation in human bronchial epithelial cells by regulating the Hedgehog pathway.
Ethics Approval
This study was approved by the Ethics Committee of Fuzhou No.1 Hospital Affiliated with Fujian Medical UniversityHow to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2023.3908
https://doi.org/10.4081/ejh.2023.3908






