HPV infection upregulates the expression of ZNT-1 in condyloma acuminatum
Accepted: 13 April 2021
HTML: 10
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
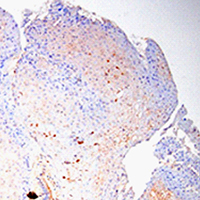
Condyloma acuminata (CA) are benign anogenital warts caused by human papillomavirus (HPV) infection with a high recurrence rate. Despite its high contagiousness, high recurrence rate and potential for malignant transformation, effective treatments for CA have not yet been developed. Accordingly, it is necessary to clarify the mechanisms underlying CA development. Zinc (Zn) is stably maintained in the weight of human body. Skin is the third most Zn-abundant tissue in the body. Zn is present as a divalent ion (Zn2+) in cells and does not need a redox reaction upon crossing the cellular membrane. Zn transporters (ZnTs; SLC30A) and Irt-like proteins (ZIPs; SLC39A) are involved in Zn2+ efflux and uptake, respectively. ZnT1 is one of the ZnTs, which associates with the development of HPV. However, the role of ZnT1 regulation in the CA caused by HPV infection remains unknown. A multigroup case-control study was designed to investigate the expression and significance of the ZnT1 in patients with CA infected with HPV and in normal vulva controls. ZnT1 was assessed by immunohistochemistry in 44 patients with CA at Zhongnan Hospital of Wuhan University 2019-2020. Samples were analyzed by paraffin embedding and sectioning and hematoxylin-eosin and immunohistochemical staining. Immunohistochemical methods detected specific, dark brown, positive staining of ZnT1 in the keratinocytes of epidermis. We verified that the expression levels of ZnT1 that interact with HPV were upregulated in the CA groups independently of genotype compared with the control group. And then we found that the HPV risk grade in CA patients has a certain correlation with ZnT1 expression. These findings showed that HPV infection upregulated the expression of ZnT1 in CA. Additionally, there were obvious differences in the expression of ZnT1 between the different HPV risk grade infection groups. The higher the HPV risk grade, the stronger the ZNT1 protein expression. This study provided new insights into the sign pathway to HPV infection.
Supporting Agencies
National Natural Science Foundation of China, Zhongnan Hospital of Wuhan UniversityDepartment of Radiation and Medical Oncology, Zhongnan Hospital, Wuhan University, Wuhan, China
Department of Radiation and Medical Oncology, Zhongnan Hospital, Wuhan University, Wuhan, China
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2021.3228
https://doi.org/10.4081/ejh.2021.3228






