Antioxidant support to ameliorate the oxaliplatin-dependent microglial alteration: morphological and molecular study
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Accepted: 20 October 2021
Authors
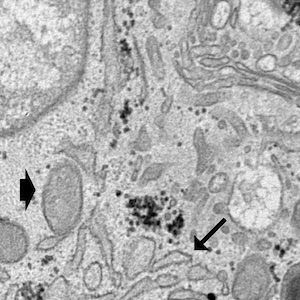
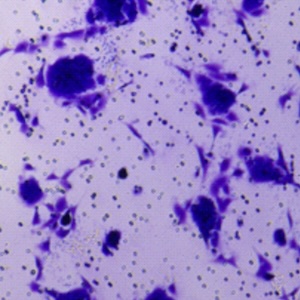
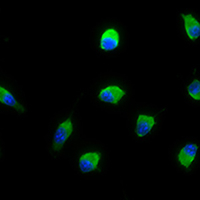
Oxaliplatin is a third-generation chemotherapy drug mainly used for colorectal cancer treatment. However, it is also known to trigger neuropathy whose underlying neurobiological mechanisms are still under investigation and currently available treatments show limited efficacy. It is now established that neurons are not the only cell type involved in chronic pain and that glial cells, mainly astrocytes and microglia, are involved in the initiation and maintenance of neuropathy. Among all the pathogenetic factors involved in neuropathic pain, an oxaliplatin-dependent oxidative stress plays a predominant role. In our study, the antioxidant properties of magnesium (Mg), manganese (Mn) and zinc (Zn) salts were evaluated in order to counteract microglial activation induced by oxaliplatin. The antioxidant efficacy of these metals was evaluated by the means of molecular and morphological assays on the BV-2 microglial cell line. Our data clearly show that Mg, Mn and Zn are able to prevent oxaliplatin-dependent microglial alterations by reducing both oxidative and endoplasmic reticulum stress.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.