Mammaglobin, GATA-binding protein 3 (GATA3), and epithelial growth factor receptor (EGFR) expression in different breast cancer subtypes and their clinical significance
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Accepted: 31 March 2022
Authors
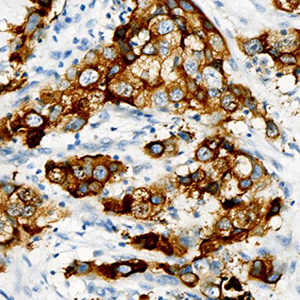
Increasing evidence has shown that mammaglobin, GATA-binding protein 3 (GATA3), and epithelial growth factor receptor (EGFR) have unique clinical implications for breast cancer subtyping and classification, as well as for breast cancer targeted therapy. It is particularly important to clarify the correlation between their expression and different molecular breast carcinoma subtypes to better understand the molecular basis of the subtypes and to identify effective therapeutic targets for the disease. This study aimed to evaluate mammaglobin, GATA3, and EGFR expression in different breast cancer subtypes, as well as their clinical significance. Subjects of the study included 228 patients with breast cancer at The First Affiliated Hospital of University of Science and Technology of China. They were divided into triple negative (TN), Luminal A, Luminal B, and HER-2 positive (HER-2.P) breast cancer groups based on molecular classification. Immunohistochemical methods were used to detect mammaglobin, GATA3, and EGFR expression in cases of different molecular subtypes before determining the correlation between protein expression and subtype. Mammaglobin and GATA3 expression levels were found to significantly vary with respect to histopathological grade, lymph node status, and molecular subtype; EGFR expression was significantly correlated with breast cancer histopathological grade and molecular subtype. For breast cancer, the expression levels of mammaglobin and GATA3, as well as mammaglobin and EGFR, were significantly correlated. In addition, there was a significantly negative correlation between the expression levels of GATA3 and EGFR in breast cancer tissue samples, especially in HER-2.P samples. These findings provide a theoretical basis for assessing breast cancer clinical prognosis based on the cancer subtype, and hence, have significant practical value.
Supporting Agencies
Anhui Natural Science Foundation (1808085MH286), Fundamental Research Funds for the Central Universities (WK91100000091)Intelligent Pathology Institute, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, China
Intelligent Pathology Institute, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, China
Intelligent Pathology Institute, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, China
Intelligent Pathology Institute, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, China
Intelligent Pathology Institute, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui; Department of Pathology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









